Published in Aesthetic Surgery Journal, the study led by Dr. Manuel Chacón-Quirós demonstrates a 3.2% complication rate in minimally invasive breast surgery.
A Paradigm Shift in Breast Surgery
The scientific journal Aesthetic Surgery Journal from Oxford Academic recently published a three-year prospective study that could transform the landscape of aesthetic breast surgery. The paper, titled “The 3-Year Results of a 100-Patient Prospective Study of Safety and Effectiveness of Mia Femtech,” was led by Dr. Manuel Chacón-Quirós, together with an international team of plastic surgeons from Costa Rica, the United Kingdom, Italy, Brazil, Belgium, Japan, and Sweden.
What is Mia Femtech™?
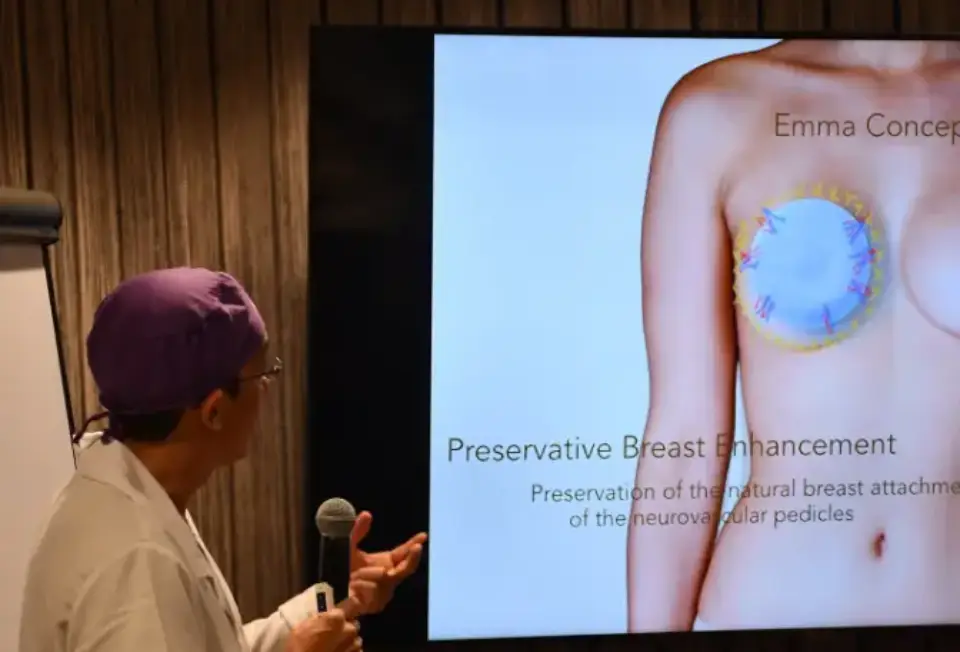
Mia Femtech™ represents an innovative approach to breast harmonization that uses an inflatable balloon and a bi-convex shaped silicone implant. Unlike conventional techniques that may compromise the natural breast structure, this minimally invasive procedure aims to increase breast volume while preserving the patient’s native tissues.
The procedure uses an axillary incision (in the armpit) and places the implant in the prepectoral plane, meaning above the pectoral muscle. This positioning is key to minimizing surgical trauma and accelerating recovery.
Study Results: Numbers That Speak for Themselves
The study, approved by an ethics committee (IRB), included 100 patients who were followed for three years between December 2020 and April 2021. The results are remarkable:
Effectiveness
- 75.3% of patients experienced an increase of one to three bra cup sizes
- 87% increase in breast satisfaction reported by patients at 3 years
- 90% of surgeons declared themselves “very satisfied” with their overall experience
- No surgeon reported being “dissatisfied”
Safety: The Grand Revelation
The overall complication rate according to Kaplan-Meier analysis was 3.2% at three years, a figure significantly lower than traditional techniques. Even more impressive, the study reported zero cases of:
- Capsular contracture (Baker grades III/IV)
- Implant rupture (also confirmed by MRI in 33 patients)
- Loss of nipple or breast sensation
- Incision-related complications
- Infection
- Seroma
- Rippling
- Hematoma
- BIA-ALCL (breast implant-associated anaplastic large cell lymphoma)
The reoperation rate was only 1% at three years, and the study maintained an exceptional 93% patient follow-up rate.
MRI Validation
A subgroup of 33 patients underwent magnetic resonance imaging, confirming implant integrity with no ruptures detected. This imaging validation further reinforces the reliability of the clinical results.
Why This Study Matters
Traditional breast augmentation surgery, although popular, faces significant challenges: social stigma, prolonged recovery times, invasion of natural breast tissue, and variable complication rates. Previous attempts to develop minimally invasive approaches had failed to demonstrate long-term safety, effectiveness, and patient satisfaction.
As explained by Dr. Manuel Chacón-Quirós, a pioneer in breast tissue preservation techniques, this study introduces a solution that systematically addresses these historical limitations with robust scientific evidence.
Study reference: Chacon-Quiros M, Sforza M, Solis-Chaves P, et al. The 3-Year Results of a 100-Patient Prospective Study of Safety and Effectiveness of Mia Femtech. Aesthetic Surgery Journal. 2025; sjaf196. https://doi.org/10.1093/asj/sjaf196